I believe in science…say the people that have probably never read a single scientific paper in their life.
If you do, you’ll realize that not all science is equal. Far from it. And thus this is a meaningless phrase.
In medical science the intervention seeks to beat the placebo.
- A list of ways to fudge the science to get statistically significant results.
- Why would you want a patented “method and system for performing a clinical trial having a reduced placebo effect”?
- What are CRO’s and what is their primary purpose?
- How regulators turned consultants use their networks and knowledge to rake in money.
- And more
Did you enjoy the podcast? Let me know by leaving a short review and be sure to hit that subscribe button so you don’t miss any future episodes!
Click the link below to see written articles and references.
Medical Monopoly Musings #61
Scientific Shenanigans in the Placebo Effect
For any drug to be approved it must be blindly tested against a placebo. Because a belief in an intervention can cause the body to heal or change, testing against placebo is done to see if a drug has a real effect more than just the placebo. (Go back to #11 to learn why we should focus on harnessing the placebo rather than simply testing against it.)
In short, if a drug performs no better than placebo then it is not helping. Or if it has more side effects than placebo then it is dangerous.
If you’re a drug company and you want to get your drug approved then it’s all about beating the placebo control group in effectiveness, and having no more side effects than the sugar pill. In the end this comes down to statistics.
If you know your outcome, winning drug approval, you can aim to hit it. Thus, the science can be manipulated in a wide variety of ways. Here are a few:
• Of course, there is straight up falsifying data. This is rare because less overt methods exist but it occurs, like it did with Merck’s Vioxx (see #7).
• Look for a secondary outcome. Instead of looking for less deaths for instance, which many people are interested in, go for some biomarker in the body that is less relevant. For instance, statins lower cholesterol but may not positively impact mortality.
• Find effects just a sub-group, but use this to promote effectiveness (as was done with Remdesivir to win approval in #44)
• Diminish side effects. (In #59 we saw how a side effect of cardiac arrest was listed as fainting, or #40 how Prozac suicides were changed to overdoses, despite normal doses being used.)
• Don’t use an inert placebo but an active one instead. (Like HCQ was put up against a placebo of vitamin C in a recent study.)
There are even more ways than these, and it is to that we turn next.
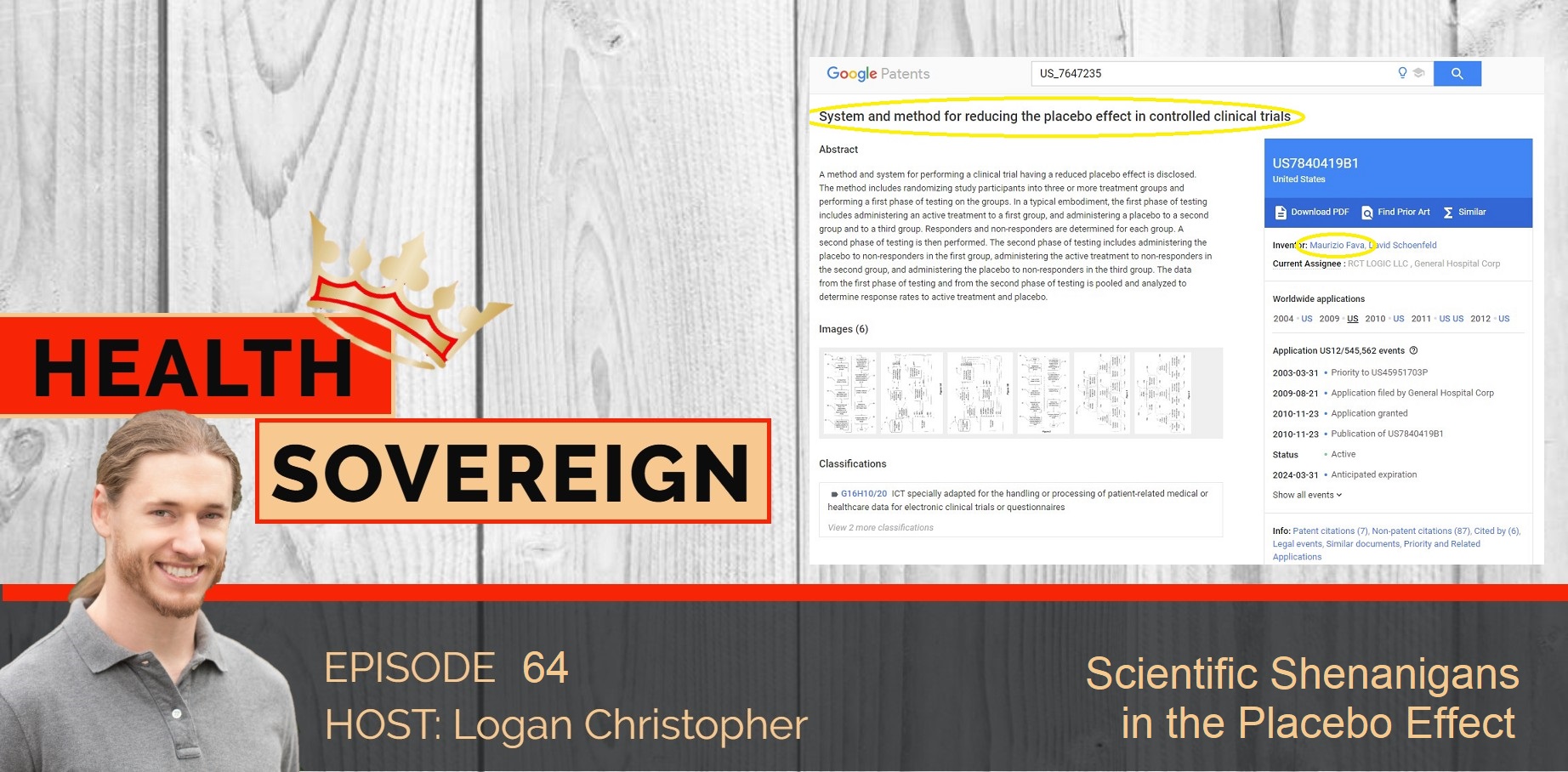
Last issue we explored Dr. Maurizio Fava who held extensive conflicts of interest while being in a scientific gatekeeper position at Massachusetts General Hospital and Harvard Medical School.
Among his lengthy conflicts was a patent section. Several of patents covered “A method and system for performing a clinical trial having a reduced placebo effect is disclosed.”
The patent filing includes, “It has been suggested that addressing the placebo response issue is one of the most important challenges facing the future of industry-sponsored psychopharmacologic drug development…The present invention comprises a system and technique for implementing a study and a related analytical plan aimed at reducing both an overall placebo response rate and a sample size requirement for clinical trials. By reducing the placebo response rate and the sample size, the present invention can, among other things, lower the expense and time required to evaluate the efficacy of new therapeutic compounds.”
Smaller sample sizes and less placebo response means that drugs have a better chance of being shown to be safe and effective.
Smaller sample sizes and less time also means less cost, which is important because, as mentioned, these are industry sponsored trials so they want faster and cheaper results.
Mentioned in the conflicts statement is that these patents are being licensed from MGH to Pharmaceutical Product Development LLC. (PPD). This company is a “leading global contract research organization.” These CRO’s do outsourced research services for pharma companies.
Their homepage states, “PPD offers proven solutions, ranging from early development to pharmacovigilance to post-approval services, to ensure your product’s success.”
Not save lives. Not heal people. Ensure product’s success…because that is really what it is all about.
Follow the money! Not only will Fava make money, but the hospital, MGH, gets funding from the patent. No wonder Fava has been promoted to the top spot there. Meanwhile, PPD, other CRO’s organizations, and consultants for drug approval such as revolving door Laughren, get paid by Big Pharma for success.
My, my…what a tangled web of conflicts we weave! It is no wonder so few people can understand how it’s possible they get away time and time again.
References:
https://jamanetwork.com/journals/jamainternalmedicine/fullarticle/416105
https://nyulangone.org/news/clinical-trial-tests-efficacy-common-antimalarial-drug-prevent-covid-19-infection
https://web.archive.org/web/20180517181848/https://mghcme.org/faculty/faculty-detail/maurizio_fava
https://patents.google.com/patent/US7840419B1/
https://patents.google.com/patent/US7647235B1/
https://patents.google.com/patent/US7983936B1/
https://www.ppd.com/